Thomas W. McGovern, MD, MAJ, MC
George W. Christopher, LTC, USAF, MC
The opinions and assertions contained herein are those of the authors and not to be considered as reflecting the views of the Department of the Army or the Department of Defense.
Key Words: biological warfare, cutaneous manifestations, hemorrhagic fever, mycotoxins, poxviruses, plague, melioidosis
INTRODUCTION
Biological warfare agents have gained attention in recent years. They have been discussed in Congress and in the medical literature, and have been the subject of frequent commentaries.[109] The mention of biological warfare often elicits a sense of deadly mystery, as summarized by a Russian journalist
"I have been gathering information on bacteriological weapons (BW) for several years. Out of all the means of mass destruction, this kind can be considered as the most mysterious."[112]
This article attempts to eliminate some of that mystery by discussing the history and background of biological weapons and by reviewing agents that cause cutaneous disease. While a biological attack could result in a made-made epidemic of unprecedented scale, the classical principles of clinical medicine and epidemiology would apply. Prompt diagnosis and early interventions could reduce morbidity and mortality, and mitigate the effects of a biological attack. In the aftermath of a biological attack, dermatologists could play a critical role in recognizing the differential diagnosis of an epidemic exanthem and alerting public health officials, leading to prompt medical and public health interventions, hopefully preventing wide-spread mortality.
History
Biological Warfare (BW) is defined as the employment of biological agents to produce casualties in man or animals or damage to plants. [91] An early BW attack took place in the Black Sea port of Kaffa (now Feodossia, Ukraine) in 1346. Rats and their fleas carried the disease to attacking Tatar soldiers. In spite, the Tatars catapulted the bodies of victims at the defending Genoese who contracted plague and left Kaffa. The same rats afflicting the Tatars likely brought disease to the Genoese.[5]
Another attempted use of biological warfare occurred between 1754 and 1767 when the British infiltrated smallpox-infested blankets to unsuspecting American Indians during the French and Indian war. Smallpox decimated the Indians, but it is unclear if the contaminated blankets or endemic disease brought by the Europeans caused these epidemics.[92] In 1932, the Japanese began a series of horrific experiments on human beings at Unit 731 outside Harbin, Manchuria, China.[92] At least 11 Chinese cities were attacked with the agents of anthrax, cholera, shigellosis, salmonella, and plague, and at least 10,000 died during their gruesome experiments.[27]
The United States started an offensive biological warfare program at Camp Detrick (today Fort Detrick) in Frederick, Maryland in 1943.[27] Ten years later, the defensive program began. By 1969, the U.S. had weaponized the agents causing anthrax, botulism, tularemia, brucellosis, Venezuelan equine encephalitis, and Q fever.[92]
|
Figures 2a, 2b: The "eight ball" one million liter test sphere at Fort Detrick, Maryland. Animals were tethered within the sphere while aerosolized agents were aerosolized. |
|
Figure 3: Anthrax pilot plant used to produce billions of anthrax spores at Fort Detrick, Maryland before the United States unilaterally ended offensive BW research in 1969. Spores were sent to other military sites where they were placed in weapons. The building is entirely free of anthrax spores and on the National Register of Historical Places. Tours frequently take visitors through this old production facility. |
These were soon destroyed after President Nixon unilaterally ended the U.S. offensive biological warfare program that year.[74] 1972 the U.S. signed the Biological Weapons Convention stating that it would never develop, produce, stockpile, acquire, or retain BW agents or the means to deliver them.[74]
Despite this convention, the development of BW weapons has continued. Controversial evidence suggests that yellow rain (trichothecene mycotoxins) attacks in Southeast Asia caused thousands of deaths between 1974 and 1981.[8] In 1978, Bulgarian dissident Georgi Markov was assassinated using an umbrella gun that shot ricin into his thigh.[92] At least 66 people died of inhalational anthrax when an aerosol of Bacillus anthracis spores was accidentally released from a BW research facility in Sverdlovsk, USSR in 1979.[92]
By 1991, the Iraqis had weaponized anthrax, botulinum toxin, and aflatoxin.[110] Fortunately, these were not used during Desert Shield or Desert Storm. The United Nations destroyed the final remains of the Iraqi offensive program in 1996.
|
Figure 1: Al Hakam Single-Cell Protein Plant. Iraq's major facility for the production of biological warfare agents. Under the watchful eye of the United Nations Special Commission, this plant was destroyed by Iraqui workers in May and June of 1996. (Photo courtesy of Davis Franz, DMV, PhD) |
Finally, in 1995, the Aum Shinrikyo cult, that released sarin nerve gas in a Japanese subway, was found to possess rudimentary biological weapons including anthrax, botulism, and Q fever.[92]
Advantages of Biological Warfare[91,105,106,109]
BW agents can cause large numbers of casualties with minimal logistical requirements. Perpetrators can escape long before BW agents cause casualties, due to the incubation periods of the agents. Weapons are easy and cheap to produce and can be used to selectively target humans, animals, or plants. The costs of conventional weapons ($2000), nuclear armaments ($800), and chemical agents ($600) would far outstrip the bargain-basement price of biological weapons ($1) to produce 50% casualties per square kilometer (1969 dollars).[91]
Agents can be easily procured from the environment, universities, biological supply houses, and clinical specimens.[105] In fact, a white supremacist (who happened to be a microbiologist) received a vial of Yersinia pestis shipped to his home by the American Type Culture Collection in Rockville, MD.[113] Common fermentation techniques used for producing antibiotics, toxoid vaccines, foods, and beverages can be used to grow large quantities of biological agents. Simple aerosol generating devices mounted on planes or trucks, as used for crop-dusting, can generate 1-5 micron particles ideal for causing infectious aerosols.[111] Aerosol particles 0.5-5 microns in diameter settle in the alveoli; larger particles are cleared by respiratory mucosa, and smaller particles float in and out of the alveoli without settling. BW agents are typically invisible in aerosol clouds and may not be detected until humans become ill. Panic would result as medical capabilities are quickly overwhelmed.
Disadvantages to using BW agents as weapons include hazards to the user, their dependence on optimal weather conditions to result in effective dispersal, and their possible inactivation by solar irradiation and other climatic conditions. BW attacks would most likely occur late at night or early in the morning when agents would be less likely to undergo inactivation by ultraviolet radiation. At these times, atmospheric temperature inversions would allow an agent cloud to travel at low altitude to cover its target.
Selecting a BW Agent[9,105,106,111]
Pathogens may be used against personnel, animals, or plants. Agents may kill or incapacitate victims. Incapacitating agents may be more effective in battle by both preventing a unit from carrying out its mission and overwhelming medical and evacuation assets. Agents with short incubation times would be most effective in a tactical setting, while those with longer incubation periods would appeal more to terrorists.[111]
Biological attacks against large populations would most likely be disseminated by aerosol. A respiratory portal of entry may cause different clinical features than naturally occurring disease (e.g. anthrax occurs mainly as a cutaneous disease in nature, but causes a rapidly lethal hemorrhagic mediastinitis when spores are inhaled).
Biological attacks could be attempted by contaminating food and water supplies, although modern water purification and the dilution effects in large volumes of water would negate the effectiveness of a water-borne attack.[109] While intact skin is an excellent barrier to most biological warfare agents, some agents, such as trichothecene mycotoxins, can penetrate the integument and cause systemic illness. Ingestion and cutaneous penetration are currently considered unimportant potential routes of exposure.[105] More unusual methods of dispersion could include releasing agents in their natural arthropod vectors.
Person-to-person transmission of several agents ( notably plague and smallpox) could perpetuate an epidemic. Nosocomial transmission could result from blood and body fluid exposures (hemorrhagic fever viruses, smallpox, and plague).
In 1970, WHO predicted that a city of 500,000 people would be devastated following an aerosol release of as little as 50 kg of BW agent (Table 1).[107]
Is There a Real Biological Warfare Threat?
Current unclassified information reveals that, despite the 1972 Geneva Biological Weapons Convention, at least seventeen countries are known or suspected of having offensive biological weapons programs.[104] Clearly, BW is a credible threat to our military, as it was during Desert Shield/Storm.[110] Terrorist use of BW agents could kill many people to create an unparalleled medical, political, and social crisis. Despite the fact the biological weapons have never been used against the United States,[111] we must prepare for a new age of terrorism.[105,106,109] Civilian health-care workers must know how to recognize a BW attack in the event of terrorist use of BW agents on civilian populations.[109]
Current U.S. Biological Warfare Policy
The U.S. government currently states that nuclear warfare would be used only as a last resort , and chemical warfare might be used to retaliate an enemy s first use of chemical agents. However, the U.S. has vowed to never use BW agents under any circumstances. All BW agent work is limited to defensive measures such as developing immunizations, detection methods, personal protective equipment, decontamination, rapid diagnostic tests, and treatments.[74]
United States Defensive Program
|
Figure 4: Entrance to Fort Detrick with headquarters building in background. |
The U.S. BW defense program is centered at Fort Detrick, MD at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID). No classified work is done; all research is open; investigators are encouraged to present their findings at scientific meetings and in peer-reviewed journals.[74] Information is regularly shared with foreign visitors and collaborators. Its mission is to conduct research to develop strategies, products, information, procedures, and training for medical defense against biological warfare agents (90%) and naturally occurring agents of military importance that require maximal containment for laboratory safety (10%).[91,105]
|
Figure 5: Front of main building at USAMRIID (United States Army Medical Research Institute of Infectious Diseases) on the grounds of Fort Detrick, Maryland. |
USAMRIID offers a biological warfare defense course that reviews agents most likely to be used by an enemy military. The agents listed in Table 2 have detailed clinical data sheets in the NATO handbook on biological warfare defense[91], are included in the Textbook of Military Medicine Volume entitled Medical Aspects of Chemical and Biological Warfare,[111] and/or are taught at the US Army s Management of Chemical and Biological Casualties Course.
AGENTS WITH CUTANEOUS MANIFESTATIONS AS PART OF A BW PRESENTATION
BACTERIAL AGENT Burkholderia pseudomallei
Burkholderia (formerly Pseudomonas) pseudomallei is a gram-negative bacillus isolated from soil, stagnant streams, ponds, rice paddies, and market produce in endemic areas and can cause epizootics in sheep, goats, swine, horses, and seals.[4,33,43,45,77] Humans contract disease from contamination of abrasions with soil but may also ingest or inhale organisms.[4,45] Melioidosis is endemic to southeast Asia and northern Australia, but it may occur anywhere between 20 degrees north and south latitudes.[4,45] It is most widespread in Thailand where it accounts for 19% of hospitalizations and 40% of deaths from community-acquired septicemia.[4] Mild or subclinical infections are common; 80% of Thai children are seropositive by age five years.[4]
Melioidosis most commonly presents as an acute pulmonary infection, but it may present as an acute localized skin infection or septicemia. Chronic suppurative infections often develop with secondary abscesses in the skin, brain, lungs, myocardium, liver, spleen, bones, lymph nodes, or eyes.[4] Melioidosis may remain latent for years. Even months of treatment with appropriate antibiotics do not necessarily eradicate the disease. Histologically, caseating granulomas as found in tuberculosis are seen. Melioidosis has been called the Great Imitator because the disease does not show any specific clinical features except perhaps the presentation of suppurative parotitis in children.[43] Fulminant respiratory failure, multiple pustular and necrotic skin lesions, or the radiologic appearance of tuberculosis without isolating any mycobacteria suggests the diagnosis of melioidosis.[4,43,45] Definitive diagnosis requires culturing organisms from blood or body fluids. No carrier state exists; recovery of organisms denotes active disease.[77]
Antibiotic treatment should be based on sensitivities. Ceftazidime has been most responsible for reducing mortality. Treatment must continue at least 30 days, but 60-150 days is recommended for pulmonary disease and 6-12 months for suppurative extrapulmonary disease.[45] Before antibiotics, 95% of patients died. The mortality rate for septicemic disease is over 50% and 20% for localized disease despite treatment. Overall, mortality is 40%. There are no available vaccines.[43,45,77]
Cutaneous Manifestations
Severe urticaria has been reported with pulmonary melioidosis.[93] Flushing and cyanosis may develop during septicemia. No cutaneous lesion, however, is specific or diagnostic of melioidosis, nor is any likely to be present with acute pulmonary disease. Inhalational melioidosis could lead to any of the skin manifestations mentioned below, but only after metatstatic abscesses to the skin formed, and this would likely take months. Many patients in endemic areas present with pustules or cutaneous abscesses associated with lymphangitis, cellulitis, or regional lymphadenitis.[77] Draining sinuses from lymph nodes or even bone may be present. Abscesses may ulcerate, and rarely, ecthyma gangrenosum-like lesions may form.
BW considerations
B. pseudomallei would most likely be delivered as an aerosol. However, its long incubation period would make it a less effective agent than anthrax. The lack of a vaccine and its high mortality despite treatment may increase its utility as a BW agent. Acute pneumonia could be confused with plague given the similar appearance of stained organisms.
BACTERIAL AGENT Yersinia pestis
Because of its high mortality (approximately 200 million deaths throughout history)[57], Yersinia pestis has attracted attention for development as a possible BW agent.
This gram-negative bacillus develops an anti-phagocytic carbohydrate protein envelope (F1 capsular antigen) during growth above 33o C.[57] A single gene encodes the Pla protease that provides both fibrinolytic and coagulase activities. At 37o C, fibrinolysis is most active; at 28o C, coagulation predominates. This enzyme helps organisms grow and remain in flea guts or spread through tissues in mammals. Other virulence factors act in concert with these such that only 2-10% of the bacteria needed to cause death in mammals at 25o C is necessary at 37o C.[57]
|
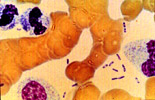
Figure 6: Wright's stain peripheral blood smear of a patient with septicemic plague demonstrating bipolar, safety-pin staining of Yersinia pestis. While Wright's stain will often demonstrate this characteristic appearance, Giemsa's and Wayson's stains most consistently highlight this pattern. (Courtesy Ken Gage, PhD., CDC, Fort Collins, CO. ) |
At least 30 types of fleas and over 200 species of mammals in 73 genera serve as reservoirs.[60] Flea infection is restricted to the alimentary canal where bacilli either are passed or stay in the midgut (stomach). There they multiply in a fibrinoid mass of blood on needle-like spines in the proventriculus. These spines aid the rupture of red blood cells and normally prevent the regurgitation of a blood meal. Such blocked fleas cannot digest their food and ultimately die. However, this state makes them ravenously hungry. In an attempt to feed, blood sucked from a mammalian host mixes with bacilli which are regurgitated back into the host. Fleas become unblocked and plague transmission ceases at temperatures above 28o C.[137] This may be caused by differential effects of Pla protease at different temperatures.[5,57]
|
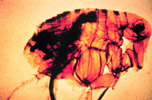
Figure 7: Here a flea is shown with a blocked proventriculus, equivalent to the gastroesophageal region in man. In nature, this flea would develop a ravenous hunger because of its inability to digest the fibrinoid mass of blood and bacteria. Ensuing biting of the nearest mammal will result in clearing of the proventriculus through regurgitation of thousands of bacteria into the bite wound. (Courtesy United States Army Environmental Hygiene Agency) |
After a flea injects a blood meal into an unsuspecting host, neutrophils and monocytes engulf the bacilli and transport them to regional lymph nodes. While the neutrophils can destroy bacilli, the monocytes cannot. In monocytes, Y. pestis multiplies and develops its anti-phagocytic capsule that prevents even neutrophils from digesting it. Bacilli then multiply in lymph nodes and the blood and travel throughout the body, but especially to the spleen, liver, lungs, and meninges.[5,57]
Every continent except Australia and Antarctica maintains enzootic foci of plague.[57] Between 1979 and 1993, 16,312 worldwide cases resulted in over 1600 deaths.[57]
Clinical Features
Most endemic plague presents with tender, erythematous lymphadenopathy, most commonly in the groin and causes bubonic plague (Greek boubon = groin). Buboes may point and drain spontaneously. [103] Bubo location is primarily a function of the region of the body in which an infected flea inoculates plague bacilli.
|
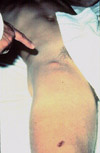
Figure 8a: Bubo of femoral lymph nodes, the most common site of erythematous, tender, swollen nodes in a plague victim. |
8b: The next most common lymph node regions involved are the inguinal, axillary (b) and cervical areas. This child has an erythematous, eroded, crusting, necrotic ulcer at the presumed primary inoculation site on the left upper quadrant. This type of lesion is uncommonly found in patients with plague. (Photos courtesy of Ken Gage, PhD., CDC, Fort Collins, CO) |
|
Figure 9: Small femoral bubo and presumed inoculation site (inferior thigh) in a patient with plague pneumonia. No chest x-ray pattern is characteristic of plague, but bilateral interstitial infiltrates are most commonly seen. (Photos courtesy of Ken Gage, PhD., CDC, Fort Collins, CO) |
A lesion is seen at the site of a flea bite no more than 10% of the time.[102] Spread to the bloodstream results in septicemic plague.[104] From the blood, the meninges may become infected. Spread to the lungs results in pneumonic plague that is rapidly fatal and transmissible. Because bacilli in pneumonic plague lesions possess an anti-phagocytic capsule, transmission by cough or sneeze can lead to death in a healthy individual within one to two days. The median infective inhaled dose is 100-500 bacilli.[105] However, as only 1-10 bacilli can infect rodents or primates via the oral, intradermal, subcutaneous, or intravenous route.[138] Respiratory droplets can be inhaled by those within two to five feet. The ensuing flu-like illness progresses rapidly to overwhelming pneumonia with cough and bloody sputum. If not treated within 24 hours of symptoms, pneumonic plague patients almost all die.[105,137] One must have a high index of suspicion to diagnose plague in the absence of buboes. Stains and cultures of blood, bubo aspirates, sputum, cerebrospinal fluid, or even skin scrapings may be helpful in isolating the organism.
The formalin-killed plague vaccine protects against bubonic, but not inhalational plague.[139,140,141] Attempts at more immunogenic live-attenuated vaccines result in no increase in immunogenicity and sporadic reversion of vaccine strains to virulent, wild type bacteria.[142] Most strains of Y. pestis are sensitive to streptomycin, gentamicin, tetracycline, chloramphenicol, trimethoprim/sulfamethoxazole, and doxycycline. Although in vitro testing has demonstrated the effectiveness of quinolones, rifampin, third-generation cephalosporins, and amoxicillin, these have not been used to any great degree in human cases.[5] The U.S. military currently requires the vaccine only for those traveling or deploying to high risk areas and for those employed in high risk occupations (entomologists or laboratory workers using Y. pestis).[20]
Cutaneous Manifestations
Terminal pneumonic and septicemic plague patients, as would be seen in a BW scenario, would develop livid cyanosis and large ecchymoses on the back.[96] Septicemia could cause petechiae, purpura, ecchymoses, and acral necrosis. [103,137]
|
Figure 10: Ecchymoses at the neck base of a girl with plague. Bandage is over the site of a prior bubo aspirate. These lesions probably gave rise to the title line of the children's nursery rhyme "ring around the rosy". (Photos courtesy of Ken Gage, PhD., CDC, Fort Collins, CO) |
No chest x-ray pattern is characteristic of plague, but bilateral interstitial infiltrates are most commonly seen.
|
Figure 11: Right-side, middle and lower lobe involvement in a patient with plague pneumonia. (Photo courtesy of Ken Gage, PhD., CDC, Fort Collins, CO) |
|
Figure 12: Rock squirrel in extremis coughing up blood-streaked sputum of pneumonic plague. (Photo courtesy of Ken Gage, PhD., CDC, Fort Collins, CO) |
Rare cases of ecthyma gangrenosum-like lesions and carbuncles due to plague have been reported.[96,103]
|
Figures 13a, b: Acral necrosis of the nose, lips, fingers (a) and toes (b) and residual ecchymoses over both forearms in a patient recovering from bubonic plague that disseminated to blood and lungs. At one time, the patient's entire body was ecchymotic. (Reprinted from McGovern TW, Friedlander AM. Plague. In: Sidell FR, Takafuji ET, Franz DR, eds. Medical Aspects of Chemical and Biological Warfare. Chapter 23 In: Zajtchuk R, Bellamy RF, eds. Textbook of Military Medicine. Washington, DC: US Department of the Army, Office of the Surgeon General, and Borden Institute; 1997:493. |
Pharyngitis associated with cervical lymphadenopathy has been reported in contacts of bubonic plague patients.[143] Of course the most common cutaneous manifestation of plague, the bubo, would not be present in a BW scenario unless the Japanese plan (discussed below) of releasing infected fleas was resurrected.
BW considerations
While Yersinia pestis would most likely be aerosolized for a BW attack, the Japanese employed a more creative approach in China during World War II. Human fleas (Pulex irritans) were multiplied and then infected with Y. pestis. These organisms were released into several Chinese cities where small epidemics of plague ensued. Normally, animal hosts die in epizootics before humans are infected, but in these cases, humans died first and then animals began dying of plague.[5,97]
Plague would most likely be transmitted as an aerosol in the event of BW.[105] The possibility of rapid death combined with possible person-to-person transmission (in contrast to anthrax) make plague an ominous BW threat. The United States studied Y. pestis as a potential offensive weapon in the 1950s. Other countries are suspected of weaponizing plague.[105]
TOXIN THREAT Trichothecene mycotoxins
Trichothecene mycotoxins ( Yellow Rain ) are the only potential BW toxins with cutaneous activity and manifestations. Mycotoxins are a diverse group of small molecular weight compounds produced by fungi.[52] They can occur at toxic levels in moldy grains and other agricultural products[8,52] and are mainly produced by members of five fungal genera: Aspergillus, Penicillium, Fusarium, Alternaria, and Claviceps.[52] Eating contaminated foodstuffs and perhaps inhaling aerosolized toxins uncommonly causes natural human or animal disease.[7,8,52]
Clinical Features
Human intoxication is rare. An entity known as alimentary toxic aleukia, reported in Russia since the 19th century, is thought to result from ingestion of mycotoxins while eating foods prepared from moldy grain. Signs and symptoms include vomiting, diarrhea, skin inflammation, leukopenia, hemorrhage, and sepsis.[8,52]
More recently, and closer to home, trichothecene mycotoxins are thought to have caused fatal pulmonary hemorrhage in Cleveland area infants. In one area of Cleveland, it may have accounted for 5% of cases of sudden infant death syndrome between 1993-95. In all cases, the fungus Stachybotrys atra was found growing in water-saturated cellulose in the walls of poorly maintained homes.[7,29,31]
Cutaneous Manifestations
At low doses (nanograms), severe skin irritation with erythema, edema, and necrosis is observed. Vesication often occurred with Yellow Rain attacks; T-2 (one of the trichothecenes) mycotoxin is estimated to be 400 times more potent than alkylating agents (mustards) in producing skin injury.[99]
|
Figure 14: Vesicles and erosions on the back of hairless guinea pigs at 1,2,7 and 14 days after application of ( bottom to top) 25, 50, 100 or 200 ng of T-2 mycotoxin in 2ul of methanol. (Reprinted from McGovern TW, Friedlander AM. Plague. In: Sidell FR, Takafuji ET, Franz DR, eds. Medical Aspects of Chemical and Biological Warfare. Chapter 23 In: Zajtchuk R, Bellamy RF, eds. Textbook of Military Medicine. Washington, DC: US Department of the Army, Office of the Surgeon General, and Borden Institute; 1997:493. |